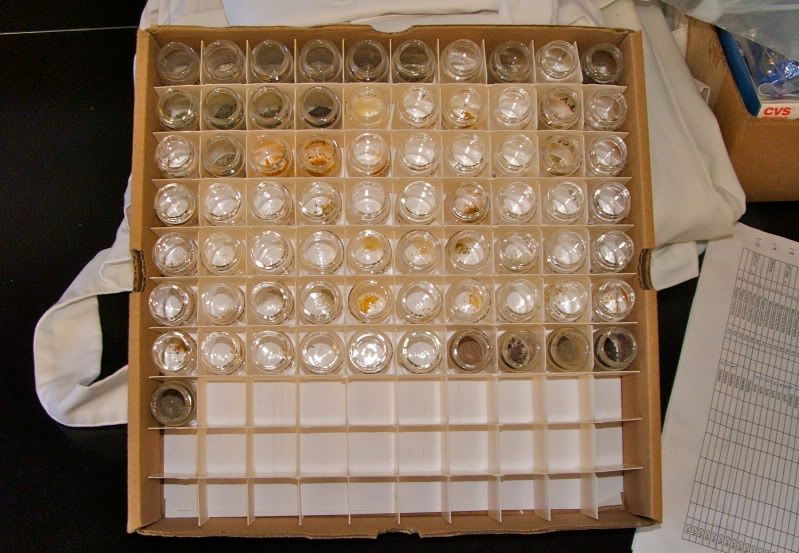
The samples are dried and ground-up bits of plants, animals, and mud from seagrass beds I've been studying in Florida's Banana River Lagoon. The isotope analysis will help me figure out how the different organisms are connected to each other in the food chain. I'll explain how, starting with a little background on what isotopes are. It has to do with neutrons.
1. From middle school chemistry, you know that atoms have a nucleus formed of tiny protons and neutrons, and the nucleus is orbited by even tinier electrons. The number of protons in the atom determine what element it is, and the number of electrons matches the number of protons so that their - and + charges balance out. For example, Carbon atoms have 6 protons and 6 electrons.
2. The number of neutrons in an atom is roughly similar to the number of protons and electrons, but there can be alternate atoms of the same element with slightly different numbers of neutrons. Those alternate versions are called "isotopes". For example, most carbon atoms are the isotope 12C, with 6 neutrons, but there is also 13C, with 7 neutrons, and 14C, with 8 neutrons.
3. Some isotopes of an element are "stable isotopes", meaning that they don't lose their extra neutrons by radioactive decay. 12C and 13C are both stable isotopes, even though 13C is relatively rare. Isotopes that do tend to lose their extra neutrons are called "radio isotopes". An example would be 14C, also known as Carbon 14, which decays to 13C. (14C is what they use in carbon dating, but that's not what I'm talking about in this post.)
4. The different stable isotopes of an atom can do all the same chemical reactions and form all the same substances because they have the same number of protons and electrons. However, the "heavy" isotopes- the ones with extra neutrons, tend to be a bit more sluggish. So the physical and chemical cycles in nature that shuffle atoms and molecules around, e.g. evaporation, photosynthesis, and the food chain, tend to concentrate heavy vs. light isotopes in different parts of the environment. It's analogous to the way shaking a box of cereal tends to concentrate the light flakes at the top and the heavy raisins at the bottom. Studying the ratio of heavy/light isotopes in a material you find in nature, anything from an antarctic ice core to a human hair sample, can tell you things about the history and formation of that material that you couldn't learn any other way. Like what temperature the atmosphere was when the ice was laid down, or whether the person was a vegetarian or not.
5. Carbon and Nitrogen are some of the key elements of life, so the stable isotopes that are most often used to study the feeding connections among living things are 13C/12C and 15N/14N. The ratio of 13C/12C is different for different kinds of plants, so you can tell approximately what kind of plants an animal got its Carbon from by comparing it's 13C/12C ratio to the ratios of the plants in the area. (Scientists can tell Americans from Europeans by our carbon isotope ratios, because Americans have a diet based mainly on corn, corn syrup, and cornfed meat and dairy, and corn has a distinctive isotope signature.) With nitrogen, the ratio of 15N/14N increases every time one organism eats another and incorporates it into it's own body. So you can tell how high an animal is in the food chain by how high its 15N/14N ratio is compared to the plants at the base of the food chain. (Eskimos have super high 15N/14N ratios, because they mainly eat meat from animals high in the marine food chain, like seals.)
Anyway, that's what I'm doing. I had to dry and grind my samples to get them ready for incineration in a machine called a "mass spectrometer", which will determine the ratio of heavy to light isotopes of Carbon and Nitrogen in each material. The Smithsonian's mass spec' facility is up in Washington DC, so I'm going to go there for a week next month.

4 comments:
Is it bad that I saw that and thought, "Wow! That's a lot of shot glasses!"
Yes it's bad, because I have to fill them with hydrochloric acid to dissolve the shell material on some of the animals. You wouldn't want to chug snail dust in acid.
But what you said gives me a good idea for a party.
That warrants an invite, right?
You have to make a shot called "Snail Dust in Acid".
I think it should involve sprinkling a little salt in white tequila and knocking it back.
I ran across your blog because I am doing the same thing in a bay of the Great Salt Lake in Utah.
Post a Comment