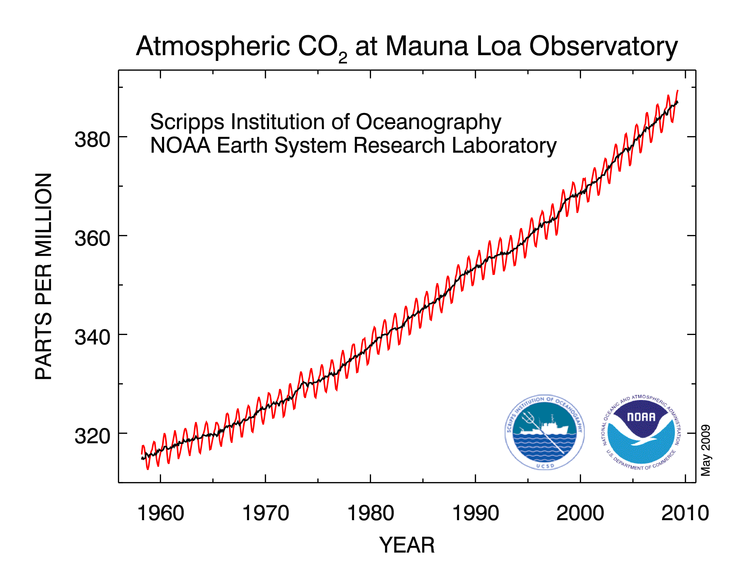
This graph shows how the CO2 level has changed since the 1950s. The little squiggles show yearly ups and downs caused by plants growing in summer (which sucks CO2 out of the atmosphere), and decaying in winter, which gives off CO2. But beneath that natural oscillation is a long-term increase that's definitely caused by humans.
Global warming isn't the only bad repercussion of the rise in CO2. There's also ocean acidification. Here's how that happens:
1. Pollution puts more CO2 into the atmosphere.
2. Some CO2 from the atmosphere dissolves into the ocean.
3. The dissolved CO2 reacts with H20 (water).
4. The reaction releases H+ ions, the cause of acidity.
Acidity is measured as "pH", and the more acid something is, the LOWER its pH is. In the 1950s the ocean water pH was 8.20. Now it's down to 8.07. That might not seem like much, but it actually means there are 25% more H+ ions in the water than there used to be. I.e., the ocean is 25% more acid than before! If the pH drops to 7.8, as is predicted for the future CO2 increase, the ocean will be 250% more acid!
That's not enough to burn your flesh or anything, but it's definitely enough to mess up the body chemistry of animals and plants that live in the ocean. Particularly, it makes it hard for them to form shells and skeletons out of calcium carbonate (CaCO3), because the CaCO3 disintegrates in acidic water. It's the same idea as when you put a tooth (also made of calcium carbonate) in a glass of coke.

Coral skeletons are made out of CaCO3, too, and the continued existence of coral reefs is dependent on the rate of accretion of new coral skeleton exceeding the rate of breakdown of old coral skeleton. Acid in the ocean is a double whammy for reefs because it slows down the growth of new coral AND increases the dissolution of old coral.
Even if we weren't doing anything else that was bad for reefs, the CO2 acidity increase could completely destroy them within a few decades. Add to that several other negative impacts humans are having on reefs, such as nutrient pollution, overfishing, and climate change, and the corals are basically screwed. It's not like a "this might happen in the future" thing, either. It's already well under way. The average coverage of live coral on Caribbean reefs used to be 50-60%. Now it's less than 10%, with most of the space taken up by dead, dissolving coral overgrown with algae, bacteria, and weedy sponges. Diving in the Dry Tortugas last month I saw some of the best-protected reefs in the Caribbean... and even there the live corals were few and far between.
The situation is definitely bad. In an excellent article for the public, reknowned coral reef expert and conservationist Dr. Nancy Knowlton put it this way: "If people don’t change the way they’re doing things, reefs as we know them will be gone by the year 2050. It’s actually really depressingly unbelievable." Yikes! Time to get way more serious about curbing emissions, and reducing nutrient pollution and overfishing. No bull.
FYI: Much of the information for this post came from a seminar given at the Smithsonian Marine Station by Dr. Chris Langdon, a coral reef expert from the University of Miami's Rosentiel School of Marine and Atmospheric Science.

8 comments:
When I was in school over population was all the rage. What happened to the their is just to my people theory? No one talks about over population anymore! Is it because only the educated rich republicans cut back? Have you ever seen a small family from Mexico? I conserve to pay for the people who are conceiving. Don't step on those corral it will hurt your feet.
Frank- It's not PC to talk about overpopulation these days, but it's still a huge problem that is the root of a lot of other problems. I've talked about it on this blog before...
http://jimbodouglass.blogspot.com/2008/02/overpopulation-killed-by-k.html
Of course, you identify one of the main issues that makes talking about overpopulation un-PC; the issue of WHO should have fewer babies. One thing that most people can agree upon, though, is that young, poor, unmarried people who don't WANT babies and can't take care of them shouldn't be having them. Better sex ed and free distribution of condoms and birth control pills could help.
Better sex ed Ha! Children that are driven by hormones and lust are not thinking. Granny always said if you don't want to be poor. Don't have children before marriage. When you get married stay married. Get a job and actually go to work. Most all the folk's having all the problems have one or more of Grannies Don'ts.
Hmm... I think we need Granny's advice AND sex ed. If a kid forgets one, there's still a chance he might remember the other. Because not all kids are completely stupid. Some are only partially stupid.
I guess you post also targets those windsurfers driving vans and pick-up trucks...windsurfers should drive hybrids
Antonio- Yeah, probably the smallest vehicle that you can still fit your stuff in and on is the best. But I reckon a van is ok if it serves multiple windsurfers... or if it doubles as a home! :)
James, I get what you're saying in your post, but even in your really bad scenario where the ocean pH goes to 7.8, it is still actually basic, not acidic. What am I missing?
BJ- Thanks for the comment!
Yeah, technically anything over pH 7.0 is considered basic. But relatively speaking, 7.8 is much "more acidic" than 8.2. The relevance to biology is that the lowered pH shifts the balance of carbonate ions (CO3--) and bicarbonate ions (HCO3-) drastically more towards bicarbonate, so there's not enough carbonate available for critters to use in calcium carbonate formation. And calcium carbonate that's already formed is more prone to dissolve.
http://www.eoearth.org/article/Marine_carbonate_chemistry
Post a Comment